Montelukast Warning Updated: The Side Effects of This Drug Are Not Just “Child Rebelliousness”
Lately in clinic I meet anxious parents every day: "Doctor, after my child took montelukast sodium (Singulair) they seem like a different person. They used to be well-behaved and sensible, and now they hit people and break things at the slightest provocation, and they have nightmares at night!"
What’s even more notable is that on December 22 the National Medical Products Administration just issued an announcement requiring a unified revision of montelukast labeling — this is not a minor matter! The announcement explicitly added a warning: "Neuropsychiatric adverse reactions have been reported in patients of all ages taking montelukast, including some serious reactions such as depression and suicidal ideation; if the drug is not discontinued, these symptoms may persist."
In fact this really isn’t the child being "disobedient," it’s a possible drug-induced side effect! As early as 2020, the U.S. FDA added the strongest black box warning to this drug. Today, based on real outpatient cases, I’ll explain this issue thoroughly.
1. These “abnormal behaviors” are actually the drug “raising an alarm”
Many parents attribute their child’s abnormal behavior to "rebellion," but in many cases I see, the drug’s side effects are to blame, which precisely corresponds to the regulator’s newly added warning.
A 6-year-old girl once had allergic rhinitis with adenoid hypertrophy and took montelukast sodium chewable tablets as prescribed. After only 7 days of medication, she began sleepwalking! Each week she would suddenly sit up, stare blankly for a while, then lie back down to sleep. The parents didn’t suspect the drug at first; only when the rhinitis improved and the medication was stopped did the sleepwalking stop! But a year later, the parents gave the child the drug again without consulting a doctor, and 5 days later the sleepwalking recurred, each episode worse than before. After stopping the medication, she improved in 2 days and the episodes disappeared completely after 1 month; switching to loratadine, they never recurred — this is a typical neuropsychiatric adverse reaction. Fortunately the drug was stopped in time; if it had been continued, the symptoms might have persisted.
Beyond sleep problems, sudden changes in mood and behavior warrant even greater vigilance! The regulatory authority explicitly highlighted “depression, suicidal tendencies” among these serious reactions — it’s not alarmism. I also encountered a 7-year-old asthmatic child who took montelukast sodium for more than 10 months and suddenly began frequent shoulder shrugs and a crooked mouth, more pronounced at night. The parents brought the child for evaluation and were diagnosed with drug-induced tic disorder. After discontinuation, these abnormal movements only slowly disappeared over 15 months — if the current package insert warnings had been available earlier, the problem might have been detected sooner.
There are other signals that are easily overlooked: a child suddenly becoming inattentive, stuttering, or developing tremor or muscle twitches. These are all listed in the adverse effect lists of the regulatory authority and the FDA! Parents should pay more attention in daily life — children can’t always express discomfort, and these abnormalities are their “cry for help”!
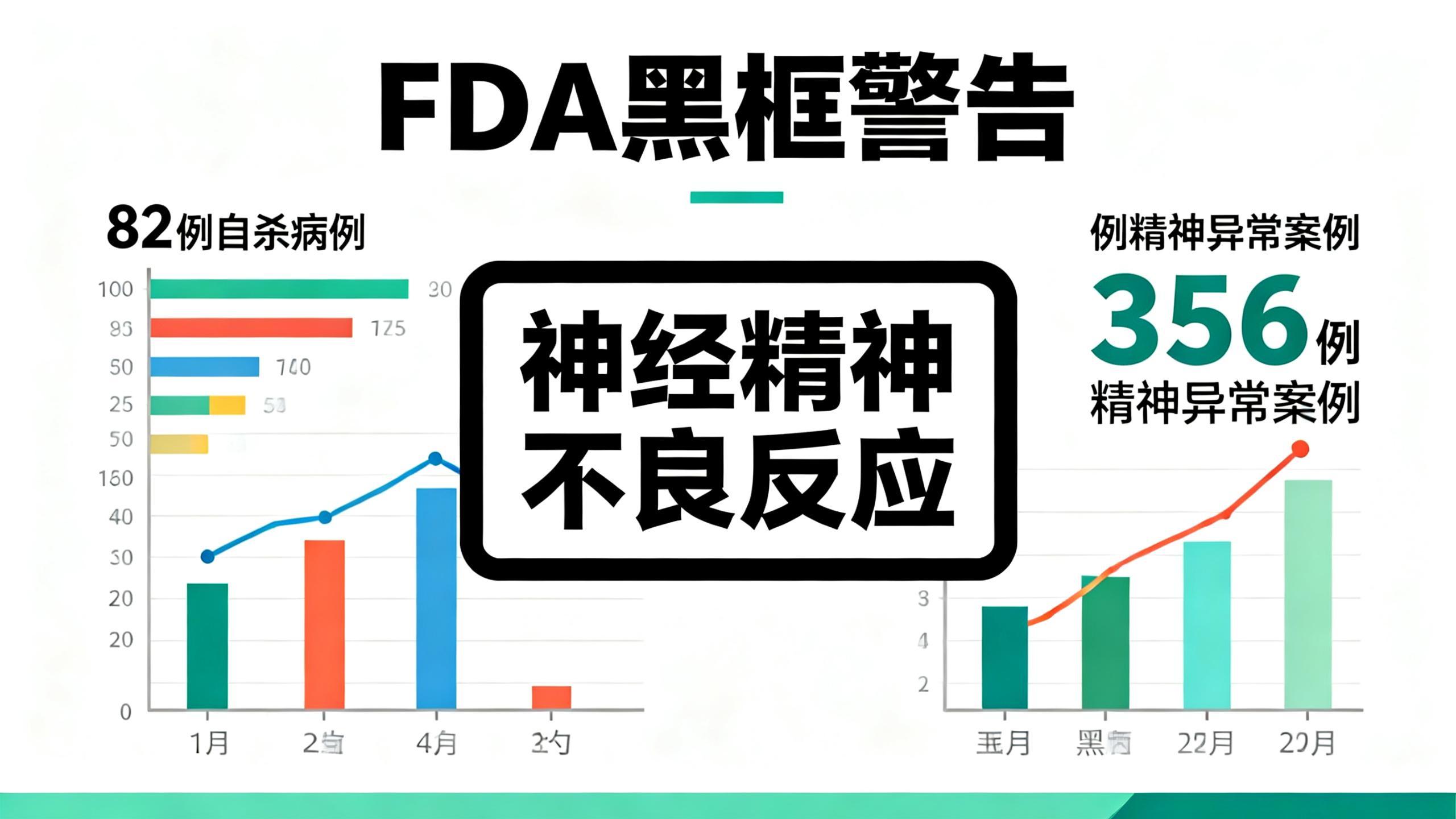
2. Why did the FDA issue the “strongest warning”? The data hide the truth
Some parents may wonder: this drug has been used for a long time, so why are domestic and international authorities now emphasizing the risks?
The FDA's black box warning did not appear out of thin air; behind it are solid risk data: from 1998 to 2019, a total of 82 suspected post-medication suicide cases were reported. Animal experiments also confirmed that montelukast sodium can directly enter brain tissue and interfere with neural function — this also explains why the drug regulator specifically warned that "symptoms may persist."
More critically, in the past many doctors and parents did not pay attention to the warnings in the package insert, often thinking "others took it fine, my child will be fine too." Only when severe adverse effects occurred did they regret it. Not only in the United States: Australia’s latest 2025 data show 356 reported cases of psychiatric abnormalities after taking the drug, of which 91 involved suicidal behavior and 10 resulted in death. These data and the drug regulator's revision notice all remind us: the risk is not far from our children, so never take it lightly!
3. Three things parents must do (Dual recommendations from the NMPA and the FDA)
As a pediatrician who deals with children every day, and in light of the drug regulatory authority's latest requirements, I summarize 3 key points to remember to avoid pitfalls:
1. Ask the doctor before taking the drug: "Is this medication absolutely necessary?"
The regulatory authority revised the label to discourage misuse of this drug. The FDA also clearly states: montelukast sodium should not be the first-line treatment for allergic rhinitis! Only consider it when intranasal steroids and antihistamines are ineffective, or the child is intolerant. Just like that sleepwalking girl — if she had initially used budesonide nasal spray (topical steroids are very safe; don’t panic at the word “steroid”) or loratadine, problems might have been avoided. The same applies to asthma treatment: a physician must assess that the benefits outweigh the risks before prescribing it to a child!
2. During treatment act as a "careful observer"; stop the drug immediately if abnormalities occur
The drug regulator specifically emphasized: "If neuropsychiatric symptoms occur during montelukast treatment, the drug should be discontinued and medical attention sought." Just like that 7-year-old boy with tic disorder—if his parents had noticed the abnormal shoulder shrugging and crooked mouth earlier and stopped the drug and sought care in time, he might not have needed 15 months to recover. If a child shows any of these signs, don't hesitate—stop the medication immediately and see a doctor:
Mood changes (sudden irritability, depression, becoming withdrawn)
Sleep disturbances (nightmares, insomnia, sleepwalking)
Abnormal behavior (hitting others, tics, stuttering)
Most symptoms will gradually improve after stopping the medication, so there's no need to be overly worried, but delaying treatment could lead to more trouble
3. Prefer safer alternatives when available
The regulatory revision does not reject this drug, but guides people to choose safer options. For example:
Allergic rhinitis: intranasal corticosteroids (budesonide, etc.), topical effect with minimal systemic side effects;
Mild to moderate allergy: oral antihistamines (cetirizine, loratadine), OTC, easy to obtain, high safety.
These are all safer than montelukast sodium!

4. Final reminder: Don’t “give up eating for fear of choking,” but use medications “precisely”
Many parents will ask after reading this: “Is this medicine completely unusable?”
No! For severe asthma or children for whom other drugs are ineffective, montelukast sodium remains an important treatment option. But two conditions must be met: strict evaluation by the physician + close observation by the parents!
If your child is currently taking this medication: do not stop it suddenly on your own (especially for children with asthma—stopping suddenly carries risks)! First take your child to see a doctor and discuss it, compare with the drug regulator’s warning language, and let the doctor decide whether to adjust the regimen.
If you haven’t started it yet: when you take your child for treatment, proactively ask the doctor, “Is there a safer alternative?”
Using medication is like buckling a child into a seatbelt; the regulator revising the package insert is like helping us check the seatbelt. Tighten the string of “monitoring for adverse effects” so the drug can truly help the child instead of becoming a health hazard!